On November 6, 2021, Neue Zurcher Zeitung (New Journal of Zurich), the Swiss newspaper of record, reported that clinics in Germany and Switzerland found defects in an astounding 50 percent of cochlear implants manufactured by Advanced Bionics. The expected failure rate for cochlear implants should be less than one percent.
The defect in the Advanced Bionics implant allows moisture to enter and short-circuit the electronics, which require removal and replacement. Delayed disclosure by Advanced Bionics put children at significant risk.
All recipients of Advanced Bionics cochlear implants losing frequencies, especially children, should be evaluated by their treating medical team. If the cochlear implant is failing to meet specifications, please call the Alexander Law Group 888.777.1776 before explanting and receiving a replacement implant. There is no charge. We can help make sure your rights are protected and pitfalls are avoided.
Surgery is necessary for everyone with a defective Advanced Bionics cochlear implant, followed by months of readapting to the new device. For young children, who have no time to spare in acquiring language, it is a tragedy to undergo replacement surgery and begin adapting to it again.
It is believed that Advance Bionics continued to sell older defective implants after receiving a warning in the summer of 2019 from German surgeons, well before issuing a warning and the subsequent February 2020 recall.
Advanced Bionics, located in Valencia, California, is a subsidiary of the Swiss hearing aid manufacturer Sonova. It is a leading producer of cochlear implants. Approximately 19,000 HiRes Ultra and HiRes Ultra 3D cochlear implants manufactured by Advance Bionics are in use.
Unlike hearing aids that amplify sound for the ear, a cochlear implant bypasses the damaged portions of the ear and stimulates the auditory nerve.
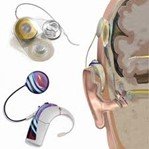
A cochlear implant is a complex electronic device that provides hearing to a profoundly deaf or severely hard-of-hearing person. The cochlear implant consists of an external part that sits behind the ear and an internal part that is surgically inserted through a flap opening and placed under the skin.
Usually, a magnet holds the external system in place next to the implanted internal system. The implant receives sound from the outside environment, processes it, and sends micro-electrical impulses by electrodes embedded in the skull and connected to the cochlea and the auditory nerve, which the brain recognizes as sound. The brain learns to recognize this signal. The wearer experiences this as “hearing.”
In a 20-minute video, a surgeon demonstrates the implanting of a cochlear implant and drills through the mastoid area of the temporal skull for inserting the electrodes into the auditory nerve.
Defects in the Ultra HiRes and Ultra HiRes 3D were first reported to Advance Bionics by the German Hearing Center at the Hanover Medical School in the summer of 2019.
The German Hearing Center is the world-renown provider of cochlear implants. Thomas Lenarz, Director of the Clinic for Ear, Nose, and Throat for the German Hearing Center, an internationally recognized specialist, reported to a Swiss journalist that “body fluid penetrates at the point where the implant should transmit signals to the auditory nerve. That leads to short circuits that damage the device.” The device’s defect was a failure of “tightness” and a repeat of previous failures involving moisture causing short-circuits.
Hearing through a cochlear implant takes time to adapt, learn or relearn. Of the 19,000 HiRes Advanced Bionics cochlear implants, children use 6,000.
For babies and young children who are deaf or severely hard-of-hearing, using a cochlear implant while young gives them early and immediate access to sound and language during the optimal period to develop full language skills and speech skills.
Babies with confirmed permanent hearing loss who receive a cochlear implant followed by intensive therapy before age 18 months are better able to hear, comprehend sound and music, and acquire spoken language than their peers who receive implants when they are older.
Studies have also shown that eligible children who receive a cochlear implant before 18 months of age develop language skills at a rate comparable to children with normal hearing, and many succeed in mainstream classrooms.
The risks of a reimplantation surgery are significant. In removing and replacing damaged electrodes, there is a risk of damaging the auditory nerve. In addition, readapting to the device takes time, which is especially critical for very young children who acquire language and develop the cognitive skills that go hand-in-hand with language development. There is no time to be lost during the optimal early years of language acquisition.
Researchers believe that Advanced Bionics continued to sell defective implants after receiving warnings in 2019 from German surgeons. That would be a repeat of previous misconduct.
In 2007 Advanced Bionics had an identical defect in previous models of its implant devices. The FDA sued Advanced Bionics for not obtaining pre-market approval for a HiRes component implant that caused excessive moisture to short the device and fail. Although Advanced Bionics recalled the implants, the defective product was sold and implanted after the recall. Advanced Bionics paid $1million to settle the FDA claims.
Several years later, a jury awarded $7.25 million to a child for the damage caused by a defective Advanced Bionics cochlear implant that delivered electrical shocks that resulted in convulsions. The jury awarded punitive damages against Advanced Bionics because it had delayed recalling the product to continue sales. That means the jury found that the company had acted willfully, intentionally, or recklessly, in conscious disregard of the safety of its customers.
Advanced Bionics’ reaction to the 2019 warning issued by German doctors was to blame the surgical techniques of the German Hearing Center. Blaming the “users” as scapegoats is a common ploy in the medical device industry when a defect is inherent in “using” the product. There is a significant difference between user failure that causes injuries and deaths and those due to a defective product that causes damage merely as a result of “using” it.
That was our experience with the Johnson & Johnson’s surgical stapler in which J&J blamed adverse outcomes on surgeons before it was recalled for difficulties in firing the stapler that caused it to jam, resulting in serious personal injuries.
Medtronic, a global producer of medical devices and famous for cutting-edge pacemaker technologies, scapegoated Mayo Clinic surgeons when its defective heart defibrillator leads were causing deaths.
The Medtronic Fidelis Sprint pacemaker leads never received a full FDA review before being implanted in patients. Multiple versions of this product evolved over 15 years. Medtronic filed a “grandfather” amendment to the original approval issued for its first pacemaker lead in each case. The company made 37 modifications under the FDA’s Pre-Market Approval [PMA] regulations, resulting in substantially different pacemaker leads that easily broke. Facing a host of broken leads and the liability for deaths and replacement surgeries, Medtronic blamed surgeons for the failure of its defective wires.
Surgeons at the Mayo Clinic were very unhappy being accused by Medtronic. As leaders in transplant surgeries, the Mayo surgeons were adamant that their technique was not in question after performing thousands of pacemaker surgeries; the problem was defective leads that readily fractured. They refused to use them. That led to a recall by Medtronic.
Advanced Bionics initiated a recall of its implants on February 17, 2020. Do not give Advanced Bionics great credit for self-initiation. That is how all recalls begin in the U.S. under an arcane regulatory system that relies entirely on culprits to police themselves. Advanced Bionics reported “fluid ingress at the electrode.” It notified recipients of “prolonged hearing degradation due to physiological fluid entering into the electrode (not the hermetic seal of the device body) and causing interruption of stimulation that can negatively affect device performance.”
As soon as Advanced Bionics learned of the leakage problem in mid-2019, it should have issued an immediate warning not to use the original versions of the HiRes implants.
On August 9, 2019, a German implant recipient posted on the DCIG (German Cochlear Implant Society) that the Hanover Medical School “is currently not implanting A.B. . . .” Advanced Bionics replied the same day that replacement Cls were available at MHH (Medizinische Hochschule Hannover).
The company filed a PMA with the FDA on October 1, 2019. The FDA issued its approval on December 23, 2019. That filing is consistent with full knowledge of the defective implants held by clinics in the summer of 2019. That is an admission by the company that replacements for the HiRes implants were commercially available in 2019. That is all the more reason for an immediate warning, especially for children, who have no time to spare in acquiring language. It is a tragedy to undergo a replacement surgery and begin again adapting to the replacement.
Avoiding delay to minimize intellectual development and language acquisition of children should have been the highest priority. It was not.
Contact Our San Jose, CA Faulty Medical Devices and Defective Consumer Products Attorneys For Immediate Help
Alexander Law Group, LLP is an established and highly respected personal injury law firm with substantial experience in collecting maximum for injuries caused by defective products, including defective medical devices, such as an award-winning victory in a $79,000,000 jury verdict against Johnson & Johnson for damages caused by a defective surgical stapler, major recoveries against car manufacturers for defective fuel systems and representing hundreds poisoned by toxic chemicals manufactured by the chemical industry. Our attorneys are available to answer questions and share our knowledge of the law and the results of our research and experience. Our goal as San Jose product liability lawyers is to make a difference for our clients. Every day we deal with a range of health and safety issues that most people do not encounter until after an injury occurs. As safety lawyers we are committed to providing our clients and the public with information for safer and healthier living. Call 888-777-1776 or contact us online to schedule a consultation to see how we can help you.


